The Ministry of Health (MoH), via its Chemistry Food and Drugs Division, advises of an international alert regarding contaminated cough syrups linked to multiple child fatalities.
According to the World Health Organization, Diethylene Glycol (DEG) has been reported in at least three oral liquid medicines identified in India. The affected products contain active ingredients commonly used to relieve symptoms of the common cold, flu, or cough.
The contaminated oral liquid medicines have been identified as the following:
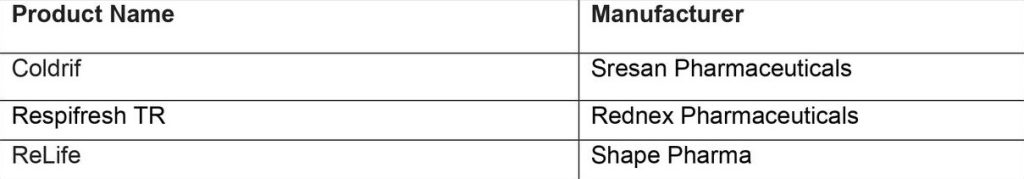
These contaminated products pose significant risks to patients and can cause severe and potentially life-threatening illness. Diethylene glycol is toxic to humans when consumed and can prove fatal. Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state and acute kidney injury that may lead to death.
While these products are not currently listed in Trinidad and Tobago’s national database, the Ministry is issuing this advisory out of an abundance of caution.
Members of the public are therefore advised to:
– Immediately discontinue use of any of the above products if in their possession.
– Consult a physician if the product has been used or if any adverse effects are experienced.
– Return the product to the point of purchase, where possible.
Additional information can be obtained by contacting the Office of the Chemistry Food and Drug Division at (868) 217-4664 Ext. 13121.